INFOWEB 18 :
Aphanius and the new discoveries in Iran, spurred from Germany with a dynamic Iranian team
From Jean H. Huber
Private address: 7 Bd Flandrin, 75116 Paris, France
M.N.H.N., Ichthyology, PARIS, France.
Paris, May 19. 2015 (updated May 16. 2018, June 16. 2020, December 17. 2024).
Dear Colleague, dear Aquarist!
The presently valid Iranian Aphanius species {in a very large sense, i.e., before 2018)
The following table is emblematic of the present situation for the Iranian Aphanius species ; our knowledge has tremendously improved during the last 3 years ; first, 5 new species have been described (arakensis, darabensis, furcatus, kavirensis, shirini) on a total of today recognised 14 valid species (resp. 16, in May 2018), then one species has been revalidated from the synonymy of sophiae and renamed (farsicus) because the old name (persicus (Jenkins, 1910)) had been found preoccupied by a fossil fish, another species has been revalidated and redescribed (pluristriatus) while it was previously considered as a junior synonym of sophiae, one species that was only known from neighboring Iraq has been collected in Iran (mesopotamicus), two species (crystallodon (Heckel in Russegger, 1847) and blanfordii (Jenkins, 1910)) have been confirmed as junior synonyms of sophiae while the status of another (punctatus (Heckel in Russegger, 1847) is still contradictory (either valid, instead of farsicus, or junior synonym of sophiae) and finally one species (mento, first discovered in Mosul, northern Iraq) has been added with certainty as native from Iran in Arvand river at frontier with Iraq (previous ambiguous reports) ; all 14 species (resp. 16, in May 2018) have been set into 3 species groups, one endemic (all species related to sophiae) another dispersed all over middle and far east (dispar) (renamed in 2 species, in May 2018) and the last (mento) as marginally present at southwestern border, each group being characterized by several palaeo-events of speciation, some very old, some quite recent ; yes, all these many new results and findings have been produced-published during the last 3 years as an exemplary cooperation between a woman German researcher, Bettina Reichenbacher, initially specialized in fish otoliths and in killifish fossils, and some young dynamic researchers (among them let's quote in alphabetical order, Mohammad Sadegh Alavi-Yeganeh, Hamid Reza Esmaeili, Zeinab Gholami, Majid Ghorbani, Bahram Kazemi, Yazdan Keivany, Mojtaba Masoudi, Golnaz Sayyadzadeh, Amir Houshang Shiva, Azad Teimori, Jafar Seyfabadi, Neda Zarei) in Iran, several of them having stayed in Munich, Germany, for long or short periods to work with modern techniques (otoliths, molecular engineering, computerized morphometrics) and others who stayed in Iran, having made extensive collections with morphological measurements on many specimens, having surveyed in depth the life history patterns of most species and also having DNA-analyzed them. Today we have 14 valid species, all described with detailed and comparative morphological and osteological characters, with molecular data, and comprehensive diagnoses.
Bravos !
And to publicize (and promote) this exceptional cooperation, I have asked Bettina Reichenbacher to write a short story on how it all started and grew and developed along time… which she has nicely done, here it is :
"The German-Iranian cooperation started in the year 2008. Bettina Reichenbacher (Professor for Palaeontology at the Ludwig-Maximilians University, Munich, Germany) participated in a joint fieldwork between ichthyologists from the Tehran and Shiraz Universities (Iran) and the Senckenberg Research Institute (Frankfurt, Germany) in Iran. There she met Hamid Reza Esmaeili (Professor for Ichthyology at Shiraz University, Iran) and also Azad Teimori and Zeinab Gholami, both master students of Hamid at that time. The group discovered that they shared a strong interest on the species of the killifish Aphanius. The initial interest of Bettina, as a palaeontologist, was examination of otolith variation between and within species of Aphanius. Such knowledge is essential to correctly interpret the taxonomic diversity of fossil killifish otoliths, but information on that issue was rare in 2008. However, a project on the species of Aphanius from Iran is providing not only otoliths, but also fish specimens that can be studied with regard to their morphological characters as well as tissues that can be used to obtain DNA sequences. So the joint project was a unique opportunity to combine all data sets in order to understand taxonomic importance and variability of characters and evolutionary relationships in the species of Aphanius. In 2009, Azad received a grant from the Iranian Ministry of Science, Research and Technology and went to Germany in order to prepare his doctoral thesis. He completed his doctorate in 2013 at LMU Munich with the best possible grade, summa cum laude. He is now Assistant Professor at Shahid Bahonar University of Kerman, Iran. Zeinab received a grant from the German Academic Exchange Service and came to Germany in 2010. First, she did a German language course (meanwhile she is speaking German fluently) and then she started preparing her doctoral thesis, which she completed successfully in 2014. Since then, she is working as a Post-Doc in the group of Bettina. Also Hamid received grants from the German Academic Exchange Service and came to Germany in summer 2010 and summer 2013 working on the same subjects and another interesting fish group (Gobiidae). All the other Iranian students that participated in the publications were master students of Hamid’s group at Shiraz University. Currently Azad, Zeinab, Hamid and Bettina are preparing several new manuscripts and also a book on the species of Aphanius in Iran, so this productive collaboration will continue".
 LIST OF CURRENT IRANIAN SPECIES WITH ASSIGNED SPECIES GROUP (updated in May 2018 and June 2020)
LIST OF CURRENT IRANIAN SPECIES WITH ASSIGNED SPECIES GROUP (updated in May 2018 and June 2020)
(notes : Aphanius dispar s.s. is not distributed in Iran any more ; Aphanius teimorii Freyhof & Yogurtçuoglu, 2020 is an unfortunate junior synonym of Aphanius hormuzensis ; see further here the new generic assignments since 2020 and 2024, in last table)
| FULL NAME | DESCRIBER(S), YEAR | SPECIES GROUP | DESCRIPTION YEAR |
|---|---|---|---|
| Aphanius arakensis | Teimori, Esmaeili, Gholami, Zarei & Reichenbacher, 2012 | [Aph.sop] | 2012 |
| Aphanius darabensis | Esmaeili, Teimori, Gholami & Reichenbacher, 2014 | [Aph.sop] | 2014 |
| Aphanius farsicus | Teimori, Esmaeili & Reichenbacher, 2011 | [Aph.sop] | 2011 |
| Aphanius furcatus | Teimori, Esmaeili, Erpenbeck & Reichenbacher, 2014 | [Aph.dis] | 2014 |
| Aphanius ginaonis | (Holly, 1929) | [Aph.dis] | 1929 |
| Aphanius hormuzensis | Teimori, Esmaeili, Hamidan & Reichenbacher, 2018 | [Aph.dis] | 2018 |
| Aphanius isfahanensis | Hrbek, Keivany & Coad, 2006 | [Aph.sop] | 2006 |
| Aphanius kavirensis | Esmaeili, Teimori, Gholami & Reichenbacher, 2014 | [Aph.sop] | 2014 |
| Aphanius mento | (Heckel in Russegger, 1843) | [Aph.men] | 1843 |
| Aphanius mesopotamicus | Coad, 2009 | [Aph.sop] | 2009 |
| Aphanius pluristriatus | (Jenkins, 1910) | [Aph.sop] | 1910 |
| Aphanius shirini | Gholami, Esmaeili, Erpenbeck & Reichenbacher, 2014 | [Aph.sop] | 2014 |
| Aphanius sophiae | (Heckel in Russegger, 1847) | [Aph.sop] | 1847 |
| Aphanius stoliczkanus | (Day, 1872) | [Aph.dis] | 1872 |
| Aphanius vladykovi | Coad, 1988 | [Aph.sop] | 1988 |
Before 2011, the scientific history of Iranian killifish was not very different from that of Turkish killifish : most species had been described in the old days (1829, 1847, 1910, 1929), all with a German influence, most species had been identified on standard morphological characters and also some micro-morphological characters (teeth, scales), even those rather recently described, all spurred by the Canadian ichthyologist and faunal specialist of the region, Brian Coad (1988, 2006, 2009), in cooperation with the pioneering Iranian researcher, Hamid Reza Esmaeili, often with ecological and karyotypical data. Only 5 species were then recognised as valid and native for Iran (dispar dispar, ginaonis, isfahanensis, sophiae, vladykovi), vs. 14 today (resp. 16, in May 2018, dispar being renamed into 2 species).
The complex palaeo-history of highly diversified genus Aphanius in the Middle East, and specifically in Iran
The final closing of the Tethys Sea near the Oligocene-Miocene boundary (approximately 16 million years ago, MYA, according to latest evidence, previously thought to be around 20-25 MYA), along the initial formation of the Red Sea, has had a major impact on the distribution of animal biodiversity ; for the first time, since the break-up of Pangaea (200 MYA), Gondwanan (African) elements have become united to Laurasian (Eurasian) elements causing the now well documented faunal and floral exchanges among those 2 continents ; the suppression of the marine seaway has also terminated the northern exchange of tropical marine elements between the Atlantic and the Indian oceans ; much less studied, although likely to have had an equally important impact on the animal biodiversity, are the geological events resulting from the closing of the Tethys Sea ; the formation and isolation of many new geological units likely resulted in the vicariant speciation of whole faunas and floras ; it has been suggested (e.g., Kosswig, 1967) that aphaniine killifishes are Tethyan relicts whose distribution can best be explained by the closing of the Tethys Sea ; indeed, all extant species, and most fossil relatives of the extant and fossil Cyprinodontiform genus Aphanius Nardo, 1827 are widely distributed along the late period Tethys Sea coastlines ; the today distribution includes coastal areas of the Mediterranean region, and coastal areas from the Gir peninsula of north-western India to northeastern Somalia including the Red Sea and the Persian Gulf, while inland distribution is restricted primarily to the Mediterranean and Near Eastern orogenic belts, including Turkey and Iran ; the geological history of the Anatolian–Zagros mountain chain (Turkey-Iran) is also complex : this region is composed of several small geological plates, most significantly in Turkey and in Iran (Iranian, Lut, Helmand and Farah plates), less so in other neighboring countries, and intervening uplifted regions such as ethnic Kurdistan, and the Lesser and Greater Caucasus mountains ; during the middle Miocene also extensive mountain building has occurred in Kurdistan, the mountainous border areas of Turkey, Iran and Iraq, and culminated in the complete emersion of the Maden complex of southeastern Turkey, around 12 MYA ; these microplates have been consolidated and compressed into one landmass by the effects of the northward drifting of Arabian Peninsula ; extensive indentation of the Arabian plate into the Iranian plate starting 10 MYA has caused the uplift of the Zagros mountains at the southern edge of the Iranian plate ; continued northeastern movement of the Arabian plate and a northerly movement of India resulted in additional mountain building by 5 MYA along the northern edge of the Iranian plateau as well as along the sutures of the Iranian, Lut, Helmand and Farah plates which compose today Iran ; according to authors, based on this currently available evidence and congruent molecular data, the various Aphanius populations occurring in isolated basins of the Zagros, Little Caucasus and the Elburz mountains, and the intervening Iranian plateau form a monophyletic group, all related to Aphanius sophiae (cryptic species) ; in the case of Iranian Aphanius, a fossil record does exist : Priem (1908) has described middle Miocene Aphanius fossils from the Lake Orumiyeh basin in northwestern Iran ; based on these fossils (and others from western Europe), the minimum estimate for the age of the genus Aphanius is 16 MYA (previously 30 MYA… however, beware, fossils only give a minimum estimate of the age of a clade) ; according to present geological and molecular evidence, largely distributed species groups have derived (separated) along the closing of the Tethys sea and parallel events, (1) the split between mento and dispar species groups is linked to the isolation of Iran from the Tethys seaway, (2) separation of furcatus ancestor from dispar ancestor is dated around 12 MYA while coastal dispersed populations of dispar such as those from Southern Iran (in May 2018, dispar being renamed into 2 species) and the Persian Gulf coast have diverged much more recently due to vicariance events in the course of the last glacial maximum during the Late Pleistocene (L.G.M., 21,000 - 18,000 years ago) and even the separation of ginaonis from dispar may even be more recent (Holocene) ; regarding the species diversity which is highest in the endorheic basins of the mountainous regions of the Iranian plateau, (3) speciation is linked with the multiple geological events starting about 12 MYA and the new data produced by the Iranian-German team (supported by otoliths and cytochrome b molecular evidence) tend to divide the inland species group into 2 major subclades : (4) the isolated vladykovi, together with shirini, darabensis, isfahanensis, characterize the older evolutionary group with a divergence time during Late Miocene–Early Pliocene (10 to 5 MYA) while (5) farsicus, arakensis, sophiae, mesopotamicus, pluristriatus, kavirensis represent the younger evolutionary group, which developed in the Late Pleistocene (100,000 – 11,700 years ago) and Early to Middle Holocene (circa 11,700 – 4,000 years ago).
All the above is very recent new or updated knowledge and it is amazing to note the progresses achieved in understanding speciation patterns (with present tools).
On the other hand it is hard to translate that evidence into taxonomy, and once again, immediately, the value (not to speak of the accuracy) of the present various species concepts sparks ; the German-Iranian team of workers has supported on several occasions the pragmatic species concept proposed in Killi-Data (artificial but useful), i.e., a species taxonomic unit is acceptable if it is to be separated from cryptic species other taxonomic units by at least 1 stable (preferably) external diagnostic morpho-meristic character (not a combination of overlapping external and internal characters !) ; according to the German-Iranian team (note 1), all species described so far from Iran can be clearly separated by molecular data, this is a base line ; (alas) several endemic Aphanius species are known that are soundly circumscribed by genetic differentiation and specific otolith morphology, whereas they differ only weakly (or only in multivariate space) with regard to morphometry and meristics ; specifically, some of the species of the above subclades show distinctive traits in color patterns, meristics, or morphometrics (i.e., dispar, ginaonis, furcatus, mento, vladykovi, isfahanensis, shirini) (in May 2018, dispar being renamed into 2 species), while others are superficially similar, show overlaps and cannot be unambiguously distinguished by external characters (i.e., farsicus, arakensis, sophiae, mesopotamicus, pluristriatus) and then we are at the limit (or in the gray area) of the species concept ; obviously that major and hot systematic issue bears no serious answer (and all supposedly final and natural answers and neo-species-concepts have fallen through with counter-examples, along time) ; the today frequent escaping strategy of naming dozens of cryptic species on limited data and material that may well, no risk, be separated by present or coming molecular data (… but who knows for sure with future tools ?) has been questioned-quarrelled several times (including by Killi-Data) ; this is not the case with the Iranian Aphanius species studied by the German-Iranian team (which does not mean that synonymizations cannot occur in the future) : all species have been studied on lots of material, morphologically and meristically, on otoliths characters and with cytochrome b data, plus, cherry on the cake, a provisional key to the species of Aphanius in Iran is provided in Esmaeili, Teimori, Gholami & Reichenbacher (2014).
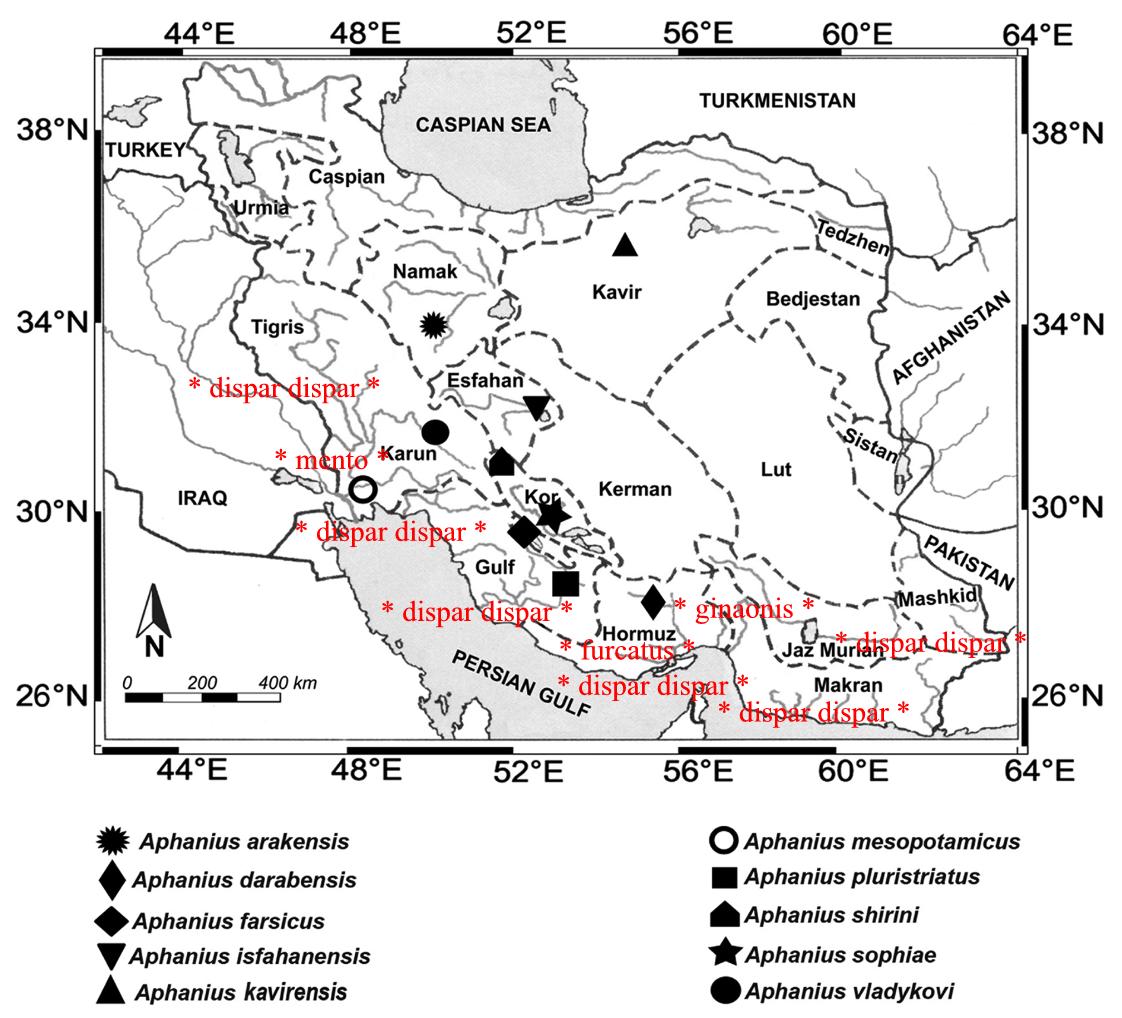 .
.
Distributional map of Aphanius species in Iran up to end of 2014 (amended by Jean Huber from Esmaieli, Teimori, Gholami and Reichenbacher, 2014) [note : the number of populations of dispar dispar has been greatly enhanced including inland, with spots in this map representing several collection sites, but in May 2018, dispar is renamed into 2 species)]
The specific and unique case of regressive evolution, Aphanius furcatus
Aphanius furcatus has been discovered lately (in September 2010) in several localities of southern Iran, in salty rivers and hot sulphuric springs in the Hormuzgan Basin, where it lives sympatrically with the related Aphanius dispar dispar (in May 2018, dispar being renamed into 2 species) ; it has been already hypothesized (Huber, Killi-Data online) that unlike for Aphanius stiassnyae in Ethiopia, a sympatric speciation (Huber, personal observation, December 2014) from a dispar ancestor or the extant dispar is not probable, because although both species are collected together, they are molecularly separated since long (ginaonis is more closely related to parapatric dispar dispar, in May 2018, dispar being renamed into 2 species)) ; however the unique specificity of furcatus is elsewhere : the species is characterized by a complete absence of scales and the reduction in the biomineralization of hard structures, particularly of the caudal skeleton and jaw teeth (i.e., it is somewhat a "soft-bone" fish, or better said a fish with less well-developed ossification) ; besides, the species is quite small (24 mm S.L.), shows a less colourful pigmentation of both male (7–11 vertical bars) and female (7–9 dark circular blotches) than dispar, and a slightly forked Caudal fin (i.e., the reverse from most Cyprinodontiformes fishes) ; ecological data : water temperature 36.7–38.8°C; water depth 8.15–35.0 cm; water speed 1 m/s; nitrate (NO3-) 1.7–2.1 mg/l; nitrite (NO2-) 0.014–0.015 mg/l; phosphate (PO4 3-) 0.21–0.36 mg/l; ammonia (NH3) 2.55–2.66 mg/l; pH 7.87–8.04; conductivity 3180–3240 µS20/cm; salinity 18.6–25.0 ppt, dissolved oxygen 7.32–7.40 mg/l ; furcatus is thriving in extreme habitats characterized by low oxygen, high water temperatures, and high salt concentration (see type locality data above) ; it appears sympatrically with dispar dispar (in May 2018, that population of dispar being renamed into hormuzensis), but the two species have different ecological preferences ; within the same river, furcatus is found along the riversides, where water temperature is high and oxygen concentration is low ; in contrast, dispar dispar (in May 2018, dispar being renamed into hormuzensis) inhabits the middle of the same river, where the water is deeper, water temperature lower, and oxygen concentration higher than along sides ; according to the describers, the ability of furcatus to survive under adverse conditions and in extreme habitats maybe responsible for its competitiveness with regard to the very successful dispar dispar (considering the wide distribution of dispar in Iran and the Persian Gulf area) (in May 2018, dispar being renamed into 2 species) ; in the habitats of furcatus, reduction of scales (and bones) saves energy and facilitates respiration, and is not disadvantageous because storage of Ca2+ (calcium) is not necessary ! Future works will deal with detailed osteological characteristics of furcatus and dispar in different habitat types (rivers and hot solphuric springs) to know more about the regressive phenomenon and ecological preferences of these species.
What do we know now on life history patterns ?
Length weight relationships have been reported for 10 species (2011, 2015) (vladykovi, sophiae, isfahanensis, dispar dispar, farsicus, ginaonis, then darabensis, furcatus, kavirensis, shirini), a rare positive situation in killifish (and for several populations, not only one per species) ; results show similar trends for all except furcatus ; maximum sizes vary according to species ranging in the wild from very small (furcatus), small (ginaonis) to more standard (sophiae, vladykovi, 6 cm) (much more in captivity).
Food is rather the same for all species ; the latest detailed survey (2015) concerns farsicus with monthly data for a population being gathered during a year with examined differences in food across seasons, sexes and sizes ; similarly to other cyprinodontids, farsicus shows sexual dimorphism and more abundance of females ; size structure and individual condition vary across seasons, with larger fish in Spring and better condition in Summer and less in Winter ; females tend to become larger than males ; no empty guts, suggesting that these fish feed all year round due to the warm climate of its native distribution ; diet is based on detritus, algae (particularly diatoms, green algae, and cyanobacteria), and small invertebrates ; seasonal variation in diet is more important than variation due to fish size and fish consume more green algae in Spring and early Summer and more diatoms and insects the rest of the year ; herbivory is considerable, similarly to quite a few other cyprinodontids, and increases with fish size, particularly because of higher consumption of green algae ; as with species composition in diet, season is more important than size in the variation of number, bio-volume, mean size, and diversity of prey captured, with higher number, richness and size of prey captured in Summer ; life expectancy is short (with older generations usually being at year age 2 or 3 in the wild), curiously a different situation from their Cyprinodon cousins in northern America who live less long.
 .
.
Aphanius typical biotope (here a shallow lake as type loc. of arakensis, else shallow rivers, in Teimori et al., 2012
What could be done next : the virgin areas of collection of eastern Iran and so-called Kurdistan and the major issues of conservation ?
Most probably the fantastic array of new species is not ended for Iran and its neighboring countries ; just in Iran, notably after the discovery of kavirensis in the northeastern part of the country, there are virgin areas of collections to the East and Southeast that are the next targets : the regions of Bedjestan, Lut, Kerman, Tedzhen, Mashkid, not to speak of the regions at borders of them in Turkmenistan, Afghanistan, Pakistan (however preliminary samplings have been disappointing, except for Kerman and Mashkid, inhabited by dispar dispar {in 2018, renamed as stoliczkanus or hormuzensis, depending on locality in southern Iran), the German-Iranian team pers. comm., April 2015)… and more importantly (for the understanding of the whole genus palaeo-history and speciation patterns), the ethnical region of Kurdistan that is -least to say-, unstable at the moment, at crossings of 3 political countries (Turkey, Iran, Iraq), with Caspian and Urmia regions in Iran (in-between the 2 hot spots of speciation of Turkey and Iran).
The other side of the medal is far less pinky : the Mediterranean region and the Near East are true biological hot spots, not only for Aphanius, i.e., containing numerous species but many of them are threatened with extinction, mainly due to human errors (e.g., over-industrialization, artificial introduction of fish as niche competitors, over-water-pumping, climate-changing desertification, road construction and pollution) ; Aphanius species are a particularly dramatic example ; the situation of Iranian populations is not encouraging ; threatened species of extinction are at least isfahanensis, ginaonis, pluristriatus, and farsicus has been recently reported as probably extinct in the wild (while most other species, notably with small distribution patterns, are vulnerable) the stupidly introduced mosquitofish (Gambusia) -stupidly because it is no better than native killifish to naturally fight malaria mosquitoes- is the n°1 enemy as a niche competitor and may well eliminate all species (as it is being the case, now, in Turkey, in España (Spain), in Israel and around, and in the Maghreb) ; besides for 2 cases, other human-caused threats are added : kavirensis is threatened by introduction of exotic carnivorous fish, Oncorhyncus mykiss, and observations on otoliths have shown that ginaonis single lacustrine population in the wild may have been poisoned by the introduction of parapatric dispar {in 2018, renamed as hormuzensis) which naturally hybridizes with ginaonis (a pity !) ; fortunately a bunch of dedicated aquarists, mainly in Europe, has tackled that conservationist issue, in close cooperation with local and European scientists, by strict programmes of maintenance and conservation in aquariums and/or outdoors artificial pools ; only future will tell if courage, tenacity and efforts in the long run will pay and save those species, known for most of them from tiny relict regions ; scientists, too, locally, are trying to alert political authorities, locally or internationally, but their voices, like everywhere in the world on that topic, are little heard by deciders and opinion-leaders ; in that context setting priorities, even if it may be seen as deceitful (over-defensive), is indeed pragmatic ; then surely the case of ginaonis would be a priority, and even if it is not endangered at this time, the case of furcatus would be another priority because these 2 species are true morphological species (the former derived recently, the latter in very old times).
New systematics of Aphaniidae after the split of genus Aphanius into 3 genera by Iranian team (updated in June 2020) ; note : Esmaeili et al., 2024, have changed the taxonomic picture by following Freyhof & Yogurtçuoglu, 2020 who split completely the hereafter defined genus Aphanius by upgrading all below subgenera to full genus status, as per last column in next table
| GENUS NAME | VALID SP. | SPECIES NAME | NEW GENUS IN 2024 |
|---|---|---|---|
| Aphaniops | Aphaniops | Aphaniops | |
| Aphaniops | Aphops. dispar | dispar | Aphaniops |
| Aphaniops | Aphops. furcatus | furcatus | Aphaniops |
| Aphaniops | Aphops. ginaonis | ginaonis | Aphaniops |
| Aphaniops | Aphops. hormuzensis | hormuzensis | Aphaniops |
| Aphaniops | Aphops. kruppi | kruppi | Aphaniops |
| Aphaniops | Aphops. richardsoni | richardsoni | Aphaniops |
| Aphaniops | Aphops. sirhani | sirhani | Aphaniops |
| Aphaniops | Aphops. stiassnyae | stiassnyae | Aphaniops |
| Aphaniops | Aphops. stoliczkanus | stoliczkanus | Aphaniops |
| Aphanius | Aphanius | ||
| Aphanius | Aph. (Aph.) almiriensis | almiriensis | Aphanius |
| Aphanius | Aph. (Anat.) anatoliae | anatoliae | Anatolichthys |
| Aphanius | Aph. (Tel.) apodus | apodus | Tellia |
| Aphanius | Aph. (Aph.) arakensis | arakensis | Esmaeilius |
| Aphanius | Aph. (Koss.) asquamatus | asquamatus | Kosswigichthys |
| Aphanius | Aph. (Tel.) baeticus | baeticus | Apricaphanius |
| Aphanius | Aph. (Anat.) chantrei | chantrei | Anatolichthys |
| Aphanius | Aph. (Anat.) danfordii | danfordii | Anatolichthys |
| Aphanius | Aph. (Aph.) darabensis | darabensis | Esmaeilius |
| Aphanius | Aph. (Aph.) farsicus | farsicus | Esmaeilius |
| Aphanius | Aph. (Aph.) fasciatus | fasciatus | Aphanius |
| Aphanius | Aph. (Anat.) fontinalis | fontinalis | Anatolichthys |
| Aphanius | Aph. (Tel.) iberus | iberus | Apricaphanius |
| Aphanius | Aph. (Anat.) iconii | iconii | Anatolichthys |
| Aphanius | Aph. (Anat.) irregularis | irregularis | Anatolichthys |
| Aphanius | Aph. (Aph.) isfahanensis | isfahanensis | Esmaeilius |
| Aphanius | Aph. (Aph.) kavirensis | kavirensis | Esmaeilius |
| Aphanius | Aph. (Anat.) maeandricus | maeandricus | Anatolichthys |
| Aphanius | Aph. (Anat.) meridionalis | meridionalis | Anatolichthys |
| Aphanius | Aph. (Aph.) mesopotamicus | mesopotamicus | Esmaeilius |
| Aphanius | Aph. (Aph.) pluristriatus | pluristriatus | Esmaeilius |
| Aphanius | Aph. (Anat.) saldae | saldae | Anatolichthys |
| Aphanius | Aph. (Tel.) saourensis | saourensis | Apricaphanius |
| Aphanius | Aph. (Aph.) shirini | shirini | Esmaeilius |
| Aphanius | Aph. (Aph.) sophiae | sophiae | Esmaeilius |
| Aphanius | Aph. (Anat.) splendens | splendens | Anatolichthys |
| Aphanius | Aph. (Anat.) sureyanus | sureyanus | Anatolichthys |
| Aphanius | Aph. (Anat.) transgrediens | transgrediens | Anatolichthys |
| Aphanius | Aph. (Anat.) villwocki | villwocki | Anatolichthys |
| Aphanius | Aph. (Aph.) vladykovi | vladykovi | Esmaeilius |
| Paraphanius | Paraphanius | ||
| Paraphanius | Paraph. mento | mento | Paraphanius |
Detailed bibliography, year by year, since 2011 and up to 2020, by the team of researchers (updated in June 2020, not updated after)
* Alavi-Yeganeh, M.S., S.J. Seyfabadi, Y. Keivany, B. Kazemi & G.P. Wallis. 2011. Length-weight Relationships in some Populations and species of Iranian Toothcarps. J. Appl. Ichthyol., 1-3, 2 tabs.
* Bakhtiyari, M., S. Kamal, A. Abdoli, H.R. Esmaeli & M. Ebrahimi. 2011. Comparison of the feeding Behaviour and Strategy of the Killifish, Aphanius sophiae Heckel, 1847, at two different Localities in Iran (Actinopterygii: Cyprinodontidae). Zoology in the Middle East, 52: 47-56.
* Teimori, A., H.R. Esmaeili & B. Reichenbacher. 2011. Aphanius farsicus, a replacement name for A. persicus (Jenkins, 1910) (Teleostei, Cyprinodontidae). Zootaxa, 3096: 53-58.
* Esmaeili, H.R., A. Teimori, Z. Gholami, N. Zarei & B. Reichenbacher. 2012. Re-Validation and Re-Description of an endemic and threatened species, Aphanius pluristriatus (Jenkins, 1910) (Teleostei, Cyprinodontidae), from southern Iran. Zootaxa, 3208: 58-67, figs.
* Golmoradizadeh, A., E. Kamrani & M.M. Sajjadi. 2012. Life History Traits of Aphanius ginaonis Holly, 1929 (Cyprinodontidae) and potential Risks of Extinction in the Geno hot spring (Iran) Population. J. Appl. Ichthyol., 28 (1): 31-33.
* Keivany, Y., M.S. Alavi-Yeganeh & S.J. Seyfabadi. 2012. A new Record confirms the Occurrence of Aphanius mesopotamicus Coad, 2009, in southwestern Iran (Actinopterygii: Cyprinodontidae). Check List, 8(2): 283-285.
* Keivany, Y. & M. Ghorbani. 2012. Distribution of Aphanius dispar dispar (Rüppell, 1829) Populations in Iran, with a new record from western Iran (Actinopterygii: Cyprinodontidae). Turk. J. Zool., 36 (6): 824-827, 3 figs., 1 tab.
* Teimori, A., H.R. Esmaeili, Z. Gholami, N. Zarei & B. Reichenbacher. 2012. Aphanius arakensis, a new species of tooth-carp (Actinopterygii, Cyprinodontidae) from the endorheic Namak Lake Basin in Iran. Zookeys, 215: 55-76, figs.
* Teimori, A., L.A.J. Jawad, L.H. Al-Kkarusi, J.N. Al-Mamry & B. Reichenbacher. 2012. Late Pleistocene to Holocene Diversification and historical Zoogeography of the Arabian killifish (Aphanius dispar) inferred from Otolith Morphology. Sci. Mar., 76 (4) : 637-645.
* Teimori, A., T. Schulz-Mirbach, H.R. Esmaeili & B. Reichenbacher. 2012. Geographical Differentiation of Aphanius dispar (Teleostei: Cyprinodontidae) from southern Iran. J. Zool. Syst. Evol. Res., 50 (4): 289-304.
* Keivany, Y. 2013. Threatened Fishes of the World: Aphanius isfahanensis Hrbek, Keivany & Coad, 2006 (Cyprinodontidae). Aqua, Journal of Ichthyology and Aquatic Biology, 19 (2): 67-70, 5 figs.
* Esmaeili, H.R., A. Teimori, G. Sayyadzadeh, M. Masoudi & B. Reichenbacher. 2014. Phylogenetic Relationships of the tooth-carp Aphanius (Teleostei: Cyprinodontidae) in the River Systems of southern and south-western Iran based on mtDNA sequences. Zool. Middle East, 60 (1) (January): 29-38, 10 figs.
* Gholami, Z., M.T. Rahimi, R. Zarei, E.B. Kia, I. Mobedi & S. Vatandoost. 2014. First report of Ichthyophthirius multifiliis (Ciliophora: Oligohymenophorea) from Aphanius dispar (Cyprinodontidae) in Iran. J. Coastal Life Medicine, 2 (6): 490-495, 2 figs., 2 tabs.
* Teimori, A., H.R. Esmaeili, D. Erpenbeck & B. Reichenbacher. 2014. A new and unique species of the genus Aphanius Nardo, 1827 (Teleostei: Cyprinodontidae) from Southern Iran: A case of regressive Evolution. Zool. Anz., 253 (4) (May): 327-337, 7 figs.
* Esmaeili, H.R., A. Teimori, Z. Gholami & B. Reichenbacher. 2014. Two new species of the tooth-carp Aphanius (Teleostei: Cyprinodontidae) and the evolutionary history of the Iranian inland and inland-related Aphanius species. Zootaxa, 3786 (3): 246-268, 5 figs.
* Alavi-Yeganeh, M.S., Y. Keivany, S.J. Seyfabadi, B. Kazemi & G.P. Wallis. 2014. Taxonomic Validity and phylogenetic Relationships of a newly-described tooth-carp, Aphanius mesopotamicus Coad, 2009 (Teleostei: Cyprinodontidae). Zootaxa, 3780 (3) (24 Mar.): 594-600.
* Gholami, Z., H.R. Esmaeili, D. Erpenbeck & B. Reichenbacher. 2014. Phylogenetic analysis of Aphanius from the endorheic Kor River Basin in the Zagros Mountains, South-western Iran (Teleostei: Cyprinodontiformes: Cyprinodontidae). J. Zool. Syst. Evol. Res., 52 (2) (May): 130-141.
* Zare, P., N. Mojtaba & S. Asghari. 2014. Reproductive biology of the Geno hot spring tooth-carp (Aphanius ginaonis Holly, 1929) in southern Iran.
* Esmaeili, H.R., M. Masoudi, G. Sayyadzadeh, H.R. Mehraban, Z. Gholami & A. Teimori. 2015. Length-Weight Relationships for four Aphanius species of Iran (Teleostei: Cyprinodontidae). Journal of Applied Ichthyology (J. Appl. Ichthyol.), doi: 10.1111/jai.12758 (online only yet)
* Gholami, Z., H.R. Esmaeili & B. Reichenbacher. 2015. New Data on the Zoogeography of Aphanius sophiae (Teleostei: Cyprinodontidae) in the Central Zagros (Southwest Iran). Limnologica, 51 (March): 70-82.
* Gholami, Z., H.R. Esmaeili, D. Erpenbeck & B. Reichenbacher. 2015. Genetic Connectivity and phenotypic Plasticity in the cyprinodont Aphanius farsicus from the Maharlu Basin, south-western Iran. Journal of Fish Biology, 86 (3) (March): 892-906.
* Alcaraz, C., Z. Gholami, H.R. Esmaeili & E. Garcia-Berthou. 2015. Herbivory and seasonal Changes in Diet of a highly endemic cyprinodontid fish (Aphanius farsicus). Environmental Biology of Fishes, DOI (online only yet).
Mansoori, A., M. Ebrahimi, A. Gholamhosseini, H.R. Esmaeili. 2016. Karyosystematics of Kol tooth-carp, Aphanius darabensis (Teleostei: Cyprinodontidae). International Journal of Aquatic Biology (IJAB), 4, No 5: pp.
Teimori, A., M. Motamedi & M. Askari Hesni. 2016. Fish Morphology and mitochondrial Phylogeny reveal Translocations of a native Aphanius Nardo, 1827 (Teleostei: Cyprinodontidae) in Iran. Iranian Jounal of Ichthyology (Iran. J. Ichthyol.), 3 (3) (September): 181-189, figs.
Masoudi, M., H.R. Esmaeili, A. Teimori, Z. Gholami, A. Gholamhosseini, G. Sayyadzadeh, Y. Keivany & B. Reichenbacher. 2016. Sympatry and possible Hybridization among species of the killifish genus Aphanius Nardo, 1827 (Teleostei: Cyprinodontidae) in Southwestern Iran. Limnologica, 59 (July): 10-20.
Jafari, O. 2016. Length-Weight Relationships and Comparison between morphological Features of Zagros tooth-carp, Aphanius vladykovi Coad, 1988 (Actinipterygii: Cyprinodontiformes) in upstreams of Karun River in Chaharmahal-o-Bakhtiari Province. Journal of Animal Researches (Iranian Biology Society), Available Online from 11 April 2016.
Esmaeili, H.R., M. Masoudi, M. Ebrahimi & A. Elmi. 2016. Review of Aphanius farsicus: a critically endangered species (Teleostei: Cyprinodontidae) in Iran. Iranian Journal of Ichthyology (Iran. J. Ichthyol.), 3(1): 1-18, figs.
Vahed, N.S., H.R. Esmaeili, M. Masoudi & M. Ebrahimi. 2017. Towards the Conservation of a critically endangered species, Aphanius farsicus : Embryogenesis and Development. Environmental Biology of Fishes (Environ. Biol. Fish), https://doi.org/10.1007/s10641-017-0691-1: 1-10.
Yaripour, S., H.R. Esmaeili, A. Gholamhosseini, M. Rezaei & S. Sadeghi. 2017. Assessment of genetic Diversity of an endangered tooth-carp, Aphanius farsicus (Teleostei: Cyprinodontiformes: Cyprinodontidae) using Microsatellite Markers. Molecular Biology Research Communications (M.B.R.C.), 6 (4):153-160.
Teimori, A., M. Motamedi & N. Manizadeh. 2017. Microstructural Characterization of the Body Key Scale Morphology in six Iranian endemic Aphanius species (Cyprinodontidae): their taxonomic and evolutionary Significance. Journal of Ichthyology, 57 (4): 533-546.
Teimori, A., M. Motamedi & M.S. Golmakan. 2017. Combining Morphology, scanning electron Microscopy, and molecular Phylogeny to evaluate the taxonomic Power of Scales in genus Aphanius Nardo, 1827 (Teleostei: Cyprinodontidae). Archives of Polish Fisheries, 25: 77-87, figs.
Aminaghaie, S. & H.R. Esmaeili. 2017. Gonad Morphology and Histology of an endemic tooth-carp, Aphanius sophiae (Heckel, 1847) from Iran. International Journal of Aquatic Biology (IJAB), 5, (3), doi: 10.7508/ijab
Teimori, A., H.R. Esmaeili, N. Hamidan & B. Reichenbacher. 2018. Systematics and historical Biogeography of the Aphanius dispar species group (Teleostei: Aphaniidae) and Description of a new species from Southern Iran. Journal of Zoological Systematics and Evolutionary Research (J. Zool. Syst. Evol. Res.), https://doi.org/10.1111/jzs.12228
Vahed, N.S., H.R. Esmaeili, M. Masoudi & B. Reichenbacher. 2018. Early Otolith Development in the critically endangered tooth-carp, Aphanius farsicus (Teleostei: Cyprinodontidae). Environmental Biology of Fishes (Environ. Biol. Fish), 101 (9): 1309-1317.
Vahed, N.S., H.R. Esmaeili, M. Masoudi & M. Ebrahimi. 2018. Embryonic and early Development of the Zagros tooth-carp, Aphanius vladykovi (Actinopterygii: Cyprinodontidae). Journal of Morphology (J. Morphol.), 279 (6): 747-756.
Motamedi, M., A. Teimori & A. Iranmanesh. 2019. Ontogenetic Pattern, morphological sexual and side Dimorphism in the saccular Otolith of a scaleless killifish Aphanius furcatus (Teleostei: Aphaniidae). Acta Zoologica (Acta Zool.), 13 pp., doi.org/10.1111/azo.12313
Esmaeili, H.R., F. Zarei, N.S. Vahed & M. Masoudi. 2019. Scale Morphology and phylogenetic Character Mapping of Scale-Surface Microstructures in sixteen Aphanius species (Teleostei: Aphaniidae). Micron 119 (April): 39-53.
Esmaeili, H.R., A. Teimori, F. Zarei & G. Sayyadzadeh. 2020. DNA Barcoding and species Delimitation of the Old World tooth-carps, family Aphaniidae Hoedeman, 1949 (Teleostei: Cyprinodontiformes). PLoS ONE, 15 (4): 17 pp., e0231717. [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0231717]
Electronic references (all species are also dealt with in Killi-Data database) :
* Coad, B.W. 2014. Freshwater fishes of Iran. Available from http://www.briancoad.com/Species%20Accounts/Contents%20new.htm (accessed, April 2015).
* Esmaeili, H.R. & J. Freyhof. 2014. Aphanius farsicus might be extinct in the wild. Newsletter of the IUCN SSC/WI Freshwater Fish Specialist Group (Saving Freshwater Fishes and Habitats) 4.Available at http://www.rufford.org/files/FFSG-Newsletter-March-2014.pdf/ (accessed April, 2014).
Note 1 : although these 3 sentences are quotes of published texts like other sentences in this newsletter, they should be illustrated with special quotes to stress the rare poise and balanced attitude of these authors which the present author feels fitting with.
P.S. : many thanks to Hamid Reza Esmaeili, Zeinab Gholami, Bettina Reichenbacher, Azad Teimori, for their comments and improvements to an earlier draft of this editorial.
In total a very important and sensitive newsletter !
Hopefully a boost to our community and a spur to speed up knowledge progress on our (beloved) fishes !
Take care and enjoy the scientific or aquaristic complexity of killifish !
Do not hesitate to ask questions for future Newsletters.
Visit frequently the website www.killi-data.org !
Thank you for your support over the years.
With my kindest regards.
Jean
I am interested in reading other Newsletters, click INFOWEB.


